COVID-19
Children take part in vaccine study as Pfizer seeks FDA approval
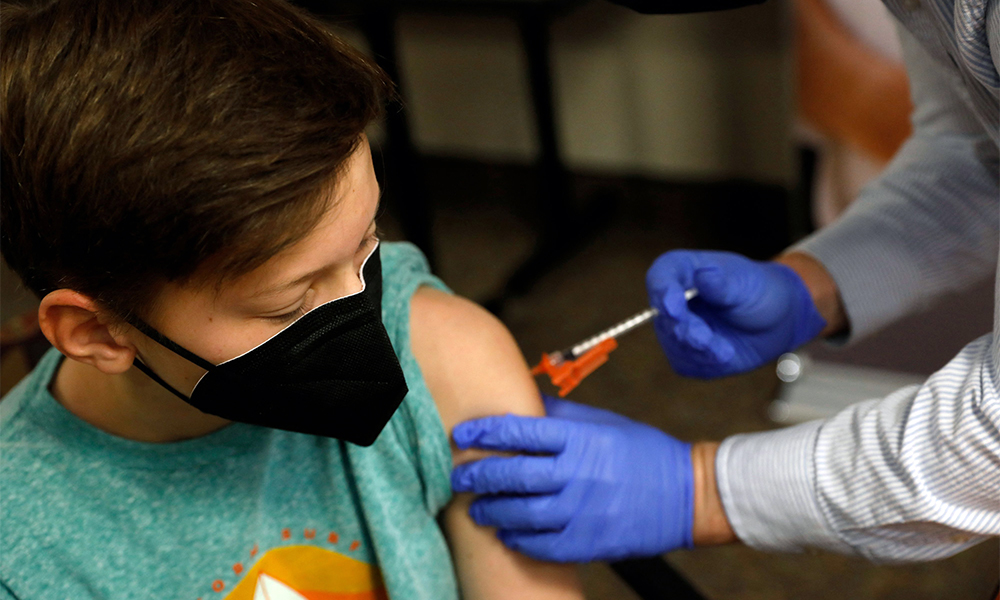
Pfizer Inc and BioNTech have asked U.S. regulators to authorize emergency use of their COVID-19 vaccine for children ages 5 to 11, a group for whom no shot is currently allowed, Pfizer said on Thursday (October 07), Reuters reported.
A trial to test vaccine safety on children took place at Duke University School of Medicine. Two of the children who took part, were 7 year old Lydia and her sister, 5 year old Bridgette.
Both of the girls were calm as they received their vaccinations, although Bridgette instructed her father to hold her hand, read the report.
The U.S. Food and Drug Administration has set a date of Oct. 26 for its panel of outside advisers to meet and discuss Pfiizer’s application, making it possible for children in this age group – numbering around 28 million – to begin receiving the two-dose Pfizer/BioNTech vaccine shortly afterward.
The vaccine already has won U.S. emergency use authorization in teens ages 12 to 15 and is fully approved by regulators for people ages 16 and up.
The Pfizer/BioNTech vaccine is one of three in use in the United States, along with the two-dose Moderna vaccine and the single-dose Johnson & Johnson version, neither of which has won full regulatory approval for any age group.
A rapid authorization of the Pfizer/BioNTech vaccine in young kids could help mitigate a potential surge of cases in the coming weeks and months, with schools open nationwide and colder weather driving activities indoors. If given regulatory authorization, the two-dose Pfizer/BioNTech vaccine would become the first COVID-19 shot made available to children 5 to 11 in the United States, Reuters reported.
COVID-19
WHO declares end to COVID global health emergency

The World Health Organization said Friday that COVID-19 no longer qualifies as a global emergency, marking a symbolic end to the devastating coronavirus pandemic that triggered once-unthinkable lockdowns, upended economies and killed millions of people worldwide.
The announcement, made more than three years after WHO declared the coronavirus an international crisis, offers some relief, if not an ending, to a pandemic that stirred fear and suspicion, hand-wringing and finger-pointing across the globe, AP reported.
The U.N. health agency’s officials said that even though the emergency phase was over, the pandemic hasn’t finished, noting recent spikes in cases in Southeast Asia and the Middle East.
WHO says thousands of people are still dying from the virus every week, and millions of others are suffering from debilitating, long-term effects.
“It’s with great hope that I declare COVID-19 over as a global health emergency,” WHO Director-General Tedros Adhanom Ghebreyesus said.
“That does not mean COVID-19 is over as a global health threat,” he said, warning that new variants could yet emerge. Tedros noted that while the official COVID-19 death toll was 7 million, the real figure was estimated to be at least 20 million.
Tedros said the pandemic had been on a downward trend for more than a year, acknowledging that most countries have already returned to life before COVID-19.
He bemoaned the damage that COVID-19 had done to the global community, saying the pandemic had shattered businesses, exacerbated political divisions, led to the spread of misinformation and plunged millions into poverty.
When the U.N. health agency first declared the coronavirus to be an international crisis on Jan. 30, 2020, it hadn’t yet been named COVID-19 and there were no major outbreaks beyond China.
More than three years later, the virus has caused an estimated 764 million cases globally and about 5 billion people have received at least one dose of vaccine.
In the U.S., the public health emergency declaration made regarding COVID-19 is set to expire on May 11, when wide-ranging measures to support the pandemic response, including vaccine mandates, will end. Many other countries, including Germany, France and Britain, dropped most of their provisions against the pandemic last year.
When Tedros declared COVID-19 to be an emergency in 2020, he said his greatest fear was the virus’ potential to spread in countries with weak health systems.
Most recently, WHO has struggled to investigate the origins of the coronavirus, a challenging scientific endeavor that has also become politically fraught.
COVID-19
COVID-19 in Iran: Nearly 900 new cases, 24 deaths recorded

The Iranian health ministry announced on Sunday that more than 890 new cases of COVID-19 have been identified across the country during the past 24 hours, adding that 24 patients have died in the same period of time, Fars News Agency reported.
“A sum of 891 new patients infected with COVID-19 have been identified in the country based on confirmed diagnosis criteria during the past 24 hours,” the Iranian Health Ministry’s Public Relations Center said on Sunday, adding, “454 patients have been hospitalized during the same time span.”
The ministry’s public relations center said 611 people infected with COVID-19 are in critical condition.
COVID-19
China says 200 million treated, pandemic ‘decisively’ beaten

China says more than 200 million of its citizens have been diagnosed and treated for COVID-19 since it lifted strict containment measures beginning in November.
With 800,000 of the most critically ill patients having recovered, China has “decisively beaten” the pandemic, according to notes from a meeting of the ruling Communist Party’s all-powerful Politburo Standing Committee presided over by President and party leader Xi Jinping, AP reported.
China enforced some of the world’s most draconian lockdowns, quarantines and travel restrictions and still faces questions about the origins of the virus that was first detected in the central Chinese city of Wuhan in late 2019. Heavy-handed enforcement prompted rare anti-government protests and took a heavy toll on the world’s second-largest economy.
The official Xinhua News Agency quoted Xi as saying that policies to control the outbreak had been “entirely correct.” The abrupt lifting in November and December of the “zero COVID” policy that had sought to eliminate all cases of the virus led to a surge in infections that temporarily overwhelmed hospitals.
Case numbers have since peaked and life has largely returned to normal, although international travel in and out of China has yet to return to pre-pandemic levels.
China is now transitioning to a post-pandemic stage after a fight against the outbreak that was “extraordinary in the extreme,” Xinhua said.
The government will continue to “optimize and adjust prevention and control policies and measures according to the times and situations with a strong historical responsibility and strong strategic determination,” Xinhua said.
-

 Sport4 days ago
Sport4 days agoOlympics finally here; What you need to know
-
 Latest News5 days ago
Latest News5 days agoOCHA reports 110 die in landmine explosions in Afghanistan every month
-

 Regional5 days ago
Regional5 days agoChina braces for twin tropical cyclones after deadly flash floods
-

 Health4 days ago
Health4 days agoHealth partners provide services 589,205 people in Afghanistan in last month
-

 Latest News4 days ago
Latest News4 days agoAfghanistan’s Hajj ministry confirms death of 27 pilgrims in Mecca and Medina
-

 Business5 days ago
Business5 days agoConference on Islamic microfinance kicks off in Kabul
-

 Sport4 days ago
Sport4 days agoACB proposes ODI fixtures against top-tiered teams
-

 Latest News4 days ago
Latest News4 days agoIslamabad claims three terrorists killed at Pakistan-Afghanistan border

























