Health
Polio vaccination campaign kicks off in 21 provinces
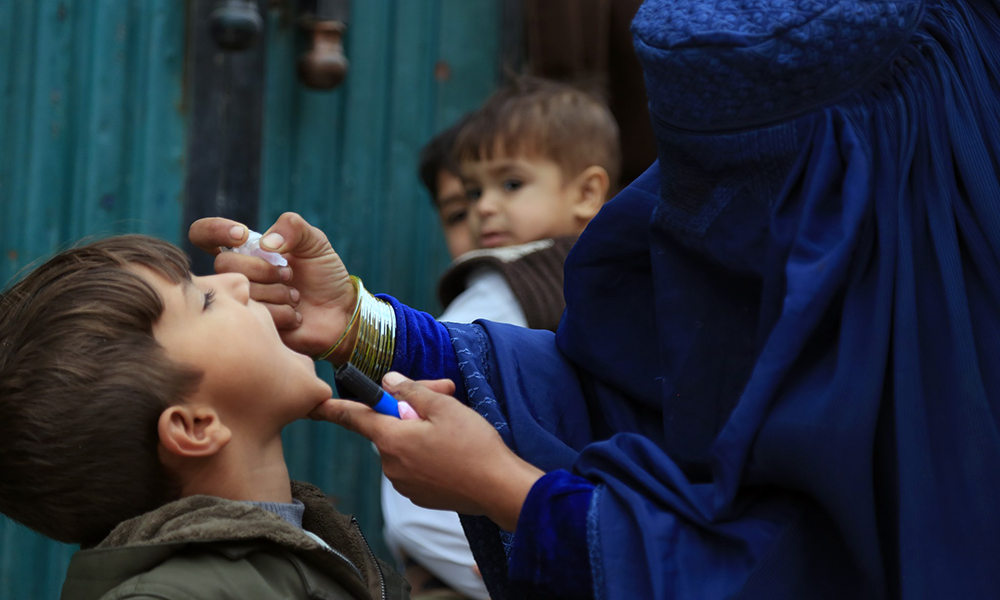
A polio vaccination campaign has been launched in 21 provinces and will reach more than 7.5 million children under the age of five, the Ministry of Public Health said.
Afghanistan and Pakistan are the only two countries in the world that still record cases of the virus on a regular basis.
However, so far this year, Afghanistan has not recorded a single case.
Health officials said that in 2023, six positive cases of polio were registered in the country.
Officials in the western region of the country say that more than 200,000 children under the age of five will be vaccinated in Herat during this four-day campaign. In the last three years, no cases of polio have been registered in this region.
Meanwhile, the World Health Organization (WHO) recently announced that there is a risk of children contracting the virus after the return of Afghan refugees from Pakistan.
Health
Health partners provide services 589,205 people in Afghanistan in last month
Based on this report, 37,342 women; 20,752 men; 22,726 girls and 15,171 boys received health services in Baghlan province.

In its June report, the World Health Organization (WHO) said that from May 10 to June 30 of this year, health cluster partners provided services to 589,205 people, including those affected by floods and other natural disasters in Afghanistan.
This organization said that in Baghlan province 95,991 people received health care services; as did 31,071 in Badakhshan; 22,448 in Ghor; 26,876 in Takhar and 9,447 in Faryab.
WHO stated these services were provided through 763 health centers in 287 districts of the country.
Based on this report, 37,342 women; 20,752 men; 22,726 girls and 15,171 boys received health services in Baghlan province.
The organization also stated that from November 1, 2023 to June 30, 2024, it provided health services to 549,154 Afghan refugees returning from neighboring countries.
In addition, WHO reported 239,009 cases of COVID since February 2020, of which 8,010 people died across Afghanistan.
The organization stated that in June this year, a total of 6,390 cases of measles were diagnosed, resulting in the death of 21 people.
It is stated in this report that “this shows a 5.9 percent decrease in the number of measles cases compared to the previous month.”
So far this year, a total of 35,210 people have been infected with measles, of which 147 people have died.
Related Stories:
Afghanistan’s health minister meets WHO chief during Switzerland visit
Health
Afghanistan’s health minister meets WHO chief during Switzerland visit
The officials from international organizations reiterated their commitment to sustained collaboration in the health sector and pledged additional support, the statement said.

Acting Health Minister Noor Jalal Jalali met with the Director-General of the World Health Organization (WHO) Tedros Adhanom Ghebreyesus during his visit to Switzerland.
The Ministry of Public Health in a statement on Saturday said that Jalali embarked on the visit to engage in high-level discussions concerning advancement and reforms in the health sector, as well as to enhance coordination with international organizations.
In addition to holding talks with the WHO chief, Jalali also participated in various international forums and engaged in deliberations with the heads of cooperating organizations on critical issues, including the control of infectious diseases in Afghanistan, the eradication of polio, and the enhancement, transparency, and standardization of health services, according to the statement.
The officials from international organizations reiterated their commitment to sustained collaboration in the health sector and pledged additional support, the statement said.
Health
A third of women in Afghanistan give birth outside a health facility: UNICEF
Just over 67 percent of births are attended by a skilled health professional, UNICEF said.

One-third of pregnant women in Afghanistan give birth outside of a health facility, the United Nations’ children agency said on Monday.
Just over 67 percent of births are attended by a skilled health professional, UNICEF said.
Although expectant mothers should have at least four antenatal visits with a skilled provider before giving birth, only one-third of women in Afghanistan do, the agency said.
“When mothers give birth outside a health facility, or without a healthcare professional to assist them, the mother’s life is at serious risk,” UNICEF said.
“If she has a birth complication, such as haemorrhage or fetal distress, there is little chance for a medical professional to intervene.”
Last month, the World Health Organization reported that Afghanistan faces a staggering daily toll of 24 maternal deaths and 167 infant deaths due to preventable causes.
Related stories:
24 mothers, 167 infants die in Afghanistan each day, WHO reports

Hundreds of women died in childbirth in past year: health ministry

-

 Sport4 days ago
Sport4 days agoOlympics finally here; What you need to know
-
 Latest News5 days ago
Latest News5 days agoOCHA reports 110 die in landmine explosions in Afghanistan every month
-

 Regional5 days ago
Regional5 days agoChina braces for twin tropical cyclones after deadly flash floods
-

 World5 days ago
World5 days agoBiden ends failing reelection campaign, backs Harris as nominee
-

 Health4 days ago
Health4 days agoHealth partners provide services 589,205 people in Afghanistan in last month
-

 Latest News4 days ago
Latest News4 days agoAfghanistan’s Hajj ministry confirms death of 27 pilgrims in Mecca and Medina
-

 Business5 days ago
Business5 days agoConference on Islamic microfinance kicks off in Kabul
-

 Sport4 days ago
Sport4 days agoACB proposes ODI fixtures against top-tiered teams



























